
© Blauplanet.com
Other sections:




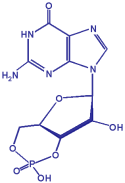
Cyclic GMP
Advanced
Created by Dr. Luis Agulló (last update on 12-6-2007)
The Molecule of Nitric Oxide
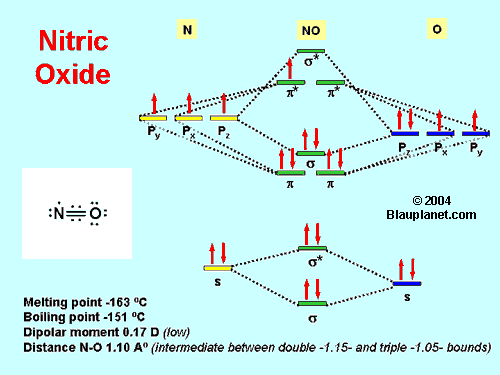
Fig. 1. The Molecule.
At room temperature and at atmospheric pressure Nitric oxide is a colorless gas with low solubility in water (its electronic structure and other chemical and physical properties are shown in Fig. 1). Its hydrophobicity and, in consequence, its high diffusion rate in biological systems allows the molecule to reach the targets before degradation. The diffusion distance of nitric oxide is in fact a matter of debate. For additional information you can read an open access paper of Vaughn et al. (1998) on this subject.
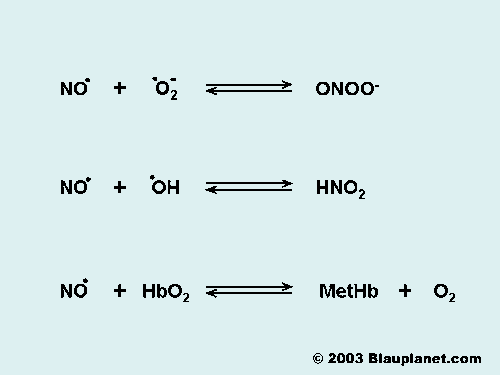
Fig. 2. The Reactions of Nitric Oxide.
Although nitric oxide is a free radical it is relatively stable. It reacts predominantly with molecules that have orbitals with unpaired electrons, which are typically other free radicals (O2- -superoxide ion-, OH. -hydroxyl ion-, .) or transition metals like heme iron (hemoglobin, myoglobin, cytochromes,.). Reactions of nitric oxide differs between in vitro and in vivo systems. In vitro systems, the main degradation product of nitric oxide is NO2- (nitrite), while in vivo the main product is NO3- (nitrate) as a consequence of the reaction of nitric oxide with hemoglobin. The main reactions of nitric oxide with physiological relevance are shown in Fig. 2.
The targets of nitric oxide depends on the environment and on the quantity produced. When concentration of nitric oxide is low (synthesis by constitutive enzymes: NOS1 and NOS3), its main target is the soluble guanylyl cyclase, the enzyme responsible for the synthesis of cyclic GMP. Nitric oxide can also modulate the function of other enzymes and of certain transcription factors (see Fig. 3).
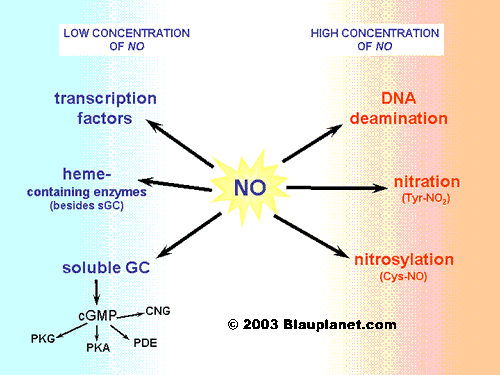
Fig. 3. Nitric oxide effects.[Based on Hanafy, Krumenacker and Murad, Med Sci Monit 2001, 7:801]. Nitric oxide may elicit a very different set of effects depending on its concentration in the tissue. At low concentrations, those normally achieved after stimulation of constitutive enzymes (NOS1 and NOS3), nitric oxide can modulate the activity of heme-containing proteins: typically guanylyl cyclase, but also important proteins of the mythocondria like cytochromes. It is also able to regulate certain transcription factors (for instance, the hypoxia inducible factor or HIF-1). At higher concentrations, nitric oxide can nitrosylate cysteine residues or produce tyrosine nitration in different proteins. In certain cases, effects of DNA deamination have been also observed.
At high concentrations (typically after induction of the inducible enzyme NOS2) nitric oxide can suffer autooxidation to produce N2O3 (dinitrogen trioxide). This compound is the main mediator of nitrosylation (formation of nitrosothiols from cysteines), that has been shown to affect protein function in many cases (e.g. p21 ras). On the other hand, as shown in Fig. 2, nitric oxide can react with O2-(superoxide ion) forming ONOO- (peroxynitrite). ONOO- can then react with tyrosine (an aminoacid with a phenol moiety) to form nitrotyrosine. This reaction, known as nitration, has also been shown to affect protein function (e.g. prostacyclin synthase).


Author: Dr. Luis Agulló (luis.agullo [at] lagullo.com).
Edited by Blauplanet.com, Cerdanyola del Vallès, Barcelona, Spain.
Webmaster: info [at] blauplanet.com. Conditions of use.