
© Blauplanet.com
Other sections:




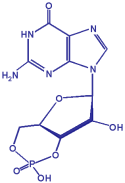
Cyclic GMP
Regulation
Created by Dr. Luis Agulló (last update on 12-6-2007)
Regulation of Endothelial Nitric Oxide Synthase
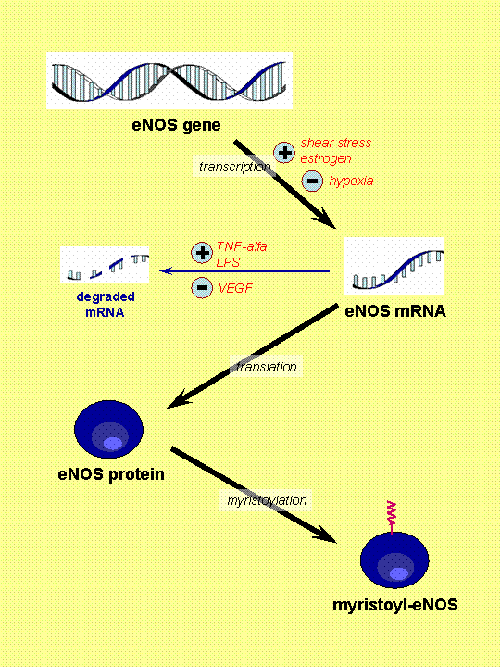
Fig. 1. eNOS regulation (part I). [Based on Govers and Rabelink, Am J Physiol 2001, 280:F193]. Here, the expression of eNOS and permanent changes of the protein (e.g. myristoylation) are shown. There are several factors that regulate the transcription of eNOS gene (shear stress, estrogen and hypoxia) and others that modulate the stability of its mRNA (tumor necrosis factor alfa or TNF-alfa, lipopolysacharide or LPS, and vascular endothelial group factor or VEGF). Myristoylation seems a critical factor to allow the final location of the enzyme at certain specific domains of the membrane.
Regulation of endothelial NO-synthase (eNOS, NOS3 or NOS-III) has been thoroughly studied during last years. It is complex and multiple regulatory pathways have been found.
Classical regulation by calcium
All NO-synthases required for its activation to be bound to a calcium regulatory protein: calmodulin. iNOS tightly binds calmodulin even at resting calcium concentrations, and then it is active once it is synthetized. However, constitutive enzymes, eNOS and nNOS, only bind calmodulin when the intracellular calcium concentration increase up to a certain value. Agents that increase intracellular calcium concentration, either by allowing calcium entrance from the outside or by stimulating calcium mobilization from intracellular stores, can activate these constitutive enzymes. In endothelial cells various substances increase intracellular calcium and in consequence NO synthesis: bradykinin, histamine, serotonin,...
Protein expression
In spite of being considered a constitutive enzyme, its expression is modulated by numerous factors. Estrogens and shear stress have been found to upregulate eNOS expression (Fig. 1).
Calcium-independent regulation
It is now clear that eNOS is also regulated by pathways that are independent on changes in the intracellular calcium concentration: its activity is acutely dependent on intracellular localization and also dependent on phosphorylation at specific aminoacids.
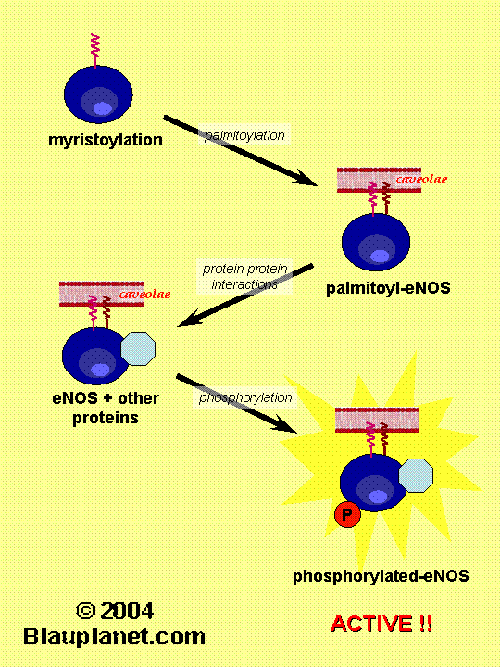
Fig. 2. eNOS regulation (part II).[Based on Govers and Rabelink, Am J Physiol 2001, 280:F193]. Besides myristoylation, eNOS protein suffers another changes such as palmitoylayion, phosphorylation and specific interactions with another proteins. After those modifications the eNOS protein is active and synthetizes NO or in some cases superoxide ion (this later circunstance can take place when the substrate, L-arginine, or tetrahydrobiopterin are deficient and has pathophysiological consequences). Then, all these non-permanent modifications of eNOS revert and eNOS is deactivated. A cycle of activation-deactivation occurs in parallel with a cycle of association and dissociation from the caveoale at the plasma membrane.
Intracellular localization
eNOS is predominantly localized in caveolae (specialized invaginations of the plasma membrane), where it is closely regulated by interaction with caveolin-1. Modifications preventing membrane localization of eNOS also result in the absence of NO synthetic activity in the intact cells. Membrane distribution is probably needed by the presence in the same localization of other proteins important for eNOS activity: the cationic amino acid transporter CAT-1 (involved in the uptake of L-arginine, substrate for NO synthesis), calcium pump and the bradykinin receptor are also present in caveolae.
Although membrane distribution is an essential requirement for eNOS activity, at plasma membrane the enzyme activity is closely regulated by caveolin-1. This intrinsic protein strongly reduces eNOS activity by interfering with calmodulin binding. Intracellular calcium increase or shear stress displace caveolin-1 and allow eNOS activation.
Membrane localization of eNOS is modulated by certain post-translational modifications:
myristoylation and palmitoylation (Fig. 2). Myristoylation distinguish eNOS from nNOS and iNOS, that are predominantly cytosolic proteins. Although in vitro activity of myristoyl-deficient eNOS is not impaired, eNOS is inactive and not localized in membrane in cells mutated in the myristoylation site.
Palmitoylation is also required for a proper localization of eNOS in the membrane. This latter modification, in contrast to myristoylation, has a rapid turnover and it has been proposed to be promoted by bradykinin stimulation and to be related with caveolin-1 displacement.
Phosphorylation
(Tyr-Phosphorylation, Ser/Thr-Phosphorylation)
Oxygen free radicals
In addition to direct regulation of NO-synthases, NO availability is also dependent on the quantity of oxygen free radicals generated by cells surrounding NO-producer cell. In fact, eNOS may generate superoxide instead of NO in certain conditions (e.g. low L-arginine levels). Whatever the origin of superoxide (eNOS, xanthine oxidase,...) this compound rapidly reacts with NO to form peroxynitrite. In certain pathological circunstances an increase in superoxide formation can be determinant in reducing NO availability.
Related Web Sites


Author: Dr. Luis Agulló (luis.agullo [at] lagullo.com).
Edited by Blauplanet.com, Cerdanyola del Vallès, Barcelona, Spain.
Webmaster: info [at] blauplanet.com. Conditions of use.